
Precast large-format gels of either type are generally not available (but casting equipment is readily available), leaving no choice but to pour your own.Īpparatus for electrophoresis with mini and midi gels (tanks, power supplies and accessories) are available that can accommodate one or more gels simultaneously. The latter are notoriously difficult to pour, even with proper gradient mixing equipment, making gel-to-gel reproducibility more difficult to achieve. But Popova, who mainly purchases precast mini gels, still prefers the sharpness of the bands in gels she has poured herself, especially for analysis or quality control in very important projects.Įven labs that cast their own homogeneous (single-percentage) gels are likely to purchase precast gradient gels. Many tout the superior performance of precast gels. It is best to check with the vendor to see what gel type it recommends for a specific application.Īs a broad generalization, academic labs usually cast their own gels, mainly for cost reasons, and labs in pharmaceutical and biotech companies prefer the convenience and purported reproducibility of (purchased) precast gels. Many such gels, such as tris-glycine, bis-tris and tris-acetate, are available from multiple sources, and others may be unique to a supplier. Selecting the right gel chemistry helps resolve high- or low-molecular weight proteins and may help prevent degradation resulting from, for example, a too low or too high pH. “This is important because we mostly express proteins for crystallization studies, and for this we need a certain quality of the recombinant proteins,” explains Izolda Popova, assistant director of the Recombinant Protein Production Core at the Chemistry of Life Processes Institute of Northwestern University. Sometimes a sample contains proteins with a wide range of MWs, in which case a gel with a gradient of polyacrylamide concentrations may be necessary-for instance, when looking for multimers, aggregates and impurities after a single purification step.
Selection charts are available with ranges of recommended concentrations. Lower-density gels are used for resolution of proteins with high MW, and higher-density gels are better for resolution of smaller proteins. The polyacrylamide (and cross-linker) concentration in the gel determines the pore size, with higher-percentage gels having smaller pores through which the proteins migrate. In a homogeneous gel with appropriate acrylamide concentration, the proteins migrate at a rate proportional to the log of their MW. Usually, the proteins are solubilized in an anionic detergent (sodium dodecyl sulfate, thus the term SDS-PAGE) that denatures them and imparts a charge proportional to their molecular weight (MW). In PAGE, a protein solution travels down a thin slab of polyacrylamide gel under the force of an electric field. The Western blotting workflow can be broadly divided into four segments:ġ) Electrophoresis or protein separation by polyacrylamide gel electrophoresis (PAGE)ģ) Probing (immunoblotting) of the proteins with labeled antibodies This guide presents information to help beginners with a basic know-how of Western blotting and to inform seasoned users about recent trends and innovations that may improve their workflow, including pros and cons of options currently available in the marketplace.
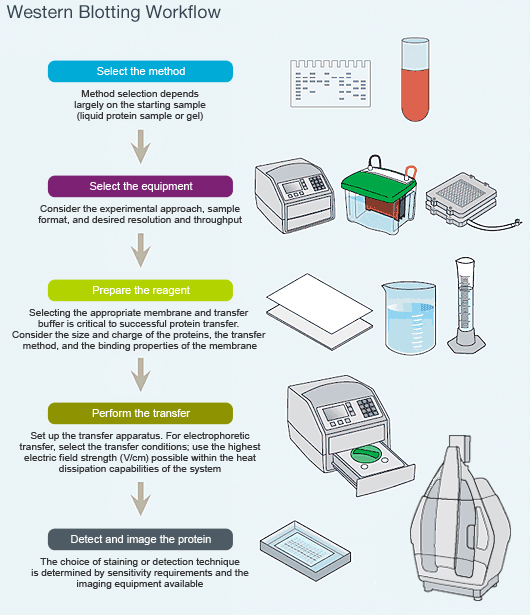
Western blotting, the immunodetection of electrophoretically separated proteins on a membrane, remains the most widely used method of detection and characterization of proteins.Īn estimated 80% to 85% of protein papers present Western blot data as part of their findings.Īlthough the basic principles remain unchanged, Western blot reagents, equipment and best practices have all evolved, sometimes significantly, since the technique’s first description in 1979.


 0 kommentar(er)
0 kommentar(er)
